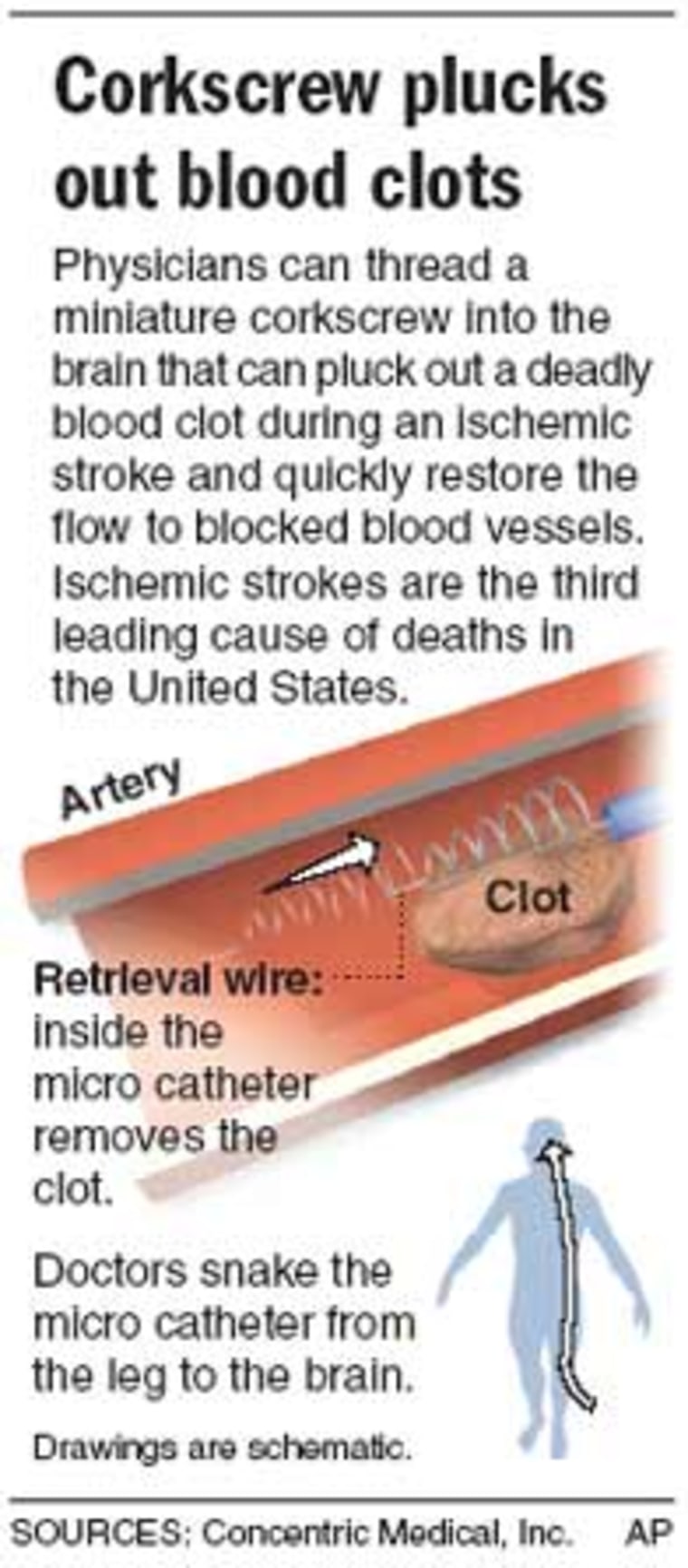
A tiny corkscrew-shaped device threaded through the body and into the brain can remove blood clots in cerebral vessels and reverse damage caused by the most common type of stroke, researchers said on Thursday.
Each year, about 700,000 Americans suffer a stroke and 88 percent of those strokes are caused by a blood clot that blocks the blood supply to the brain.
Blood clots can be dissolved using a clot-busting drug, but if it is not given intravenously within three hours of the stroke, it will not be effective. It typically takes up to two hours for the drug to dissolve the clot and open a vessel.
In the first report testing the safety and effectiveness of the nickel and titanium corkscrew, researchers at the American Stroke Association’s Stroke Conference in San Diego said it restored blood flow in 61 of 114, or 54 percent, of patients in early human trials. Researchers studied patients up to eight hours after the initial stroke symptoms who were not eligible for standard drug therapy.
Restoring blood flow in the trials reversed paralysis and other stroke symptoms, said Sidney Starkman, a physician and the co-director of the UCLA Stroke Center.
“How often do we get a chance to reverse a patient’s stroke on the table? We have had patients completely paralyzed on one side of their body, who were made normal almost instantaneously when the clot was retrieved,” he said.
Of the 61 patients whose arteries were unblocked with the device, called the Concentric Merci Retrieval System, 23 have no disability or have minor disability, such as hand writing problems, Starkman added.
The device is inserted into an artery in the groin, and then guided via standard angiography into the brain until it reaches the blood clot.
Once the device “captures” the blood clot, the device and clot are withdrawn into a larger catheter with a balloon. During the evacuation process, the balloon is briefly inflated to momentarily stop blood flow so the clots can be safely removed.
The 114 patients were from 25 centers and had an average age of 70. Forth-six percent were women. The study was funded by Concentric Medical Inc., which is trying to get U.S. regulatory approval for the device.