Several types of insulin pumps are being recalled by a medical device company because of a dangerous flaw that could affect more than 322,000 users with Type 1 diabetes.
The Food and Drug Administration identified the recall of Medtronic's MiniMed 600 Series Insulin Pumps as a "Class I" recall. This is the most serious type of recall and means that use of such products could result in serious injury or death, the Food and Drug Administration says.
Insulin pumps are devices that are designed to deliver exact doses of insulin based on a person's blood sugar levels. The devices in question are being recalled because they may be incorrectly dosing insulin. Delivering too much or too little insulin could result in life-threatening complications in people with Type 1 diabetes.
The recall is due to faulty retainer rings on the pumps, which can lead to incorrect dosing, the FDA says. Medtronic has received 26,421 complaints about the rings, resulting in at least 2,175 injuries and one death, according to the agency. The pumps affected include Medtronic MiniMed Models 630G/MMT-1715 (lots distributed from September to October 2019) and 670G/MMT-1770 (lots distributed from June 2017 to August 2019).
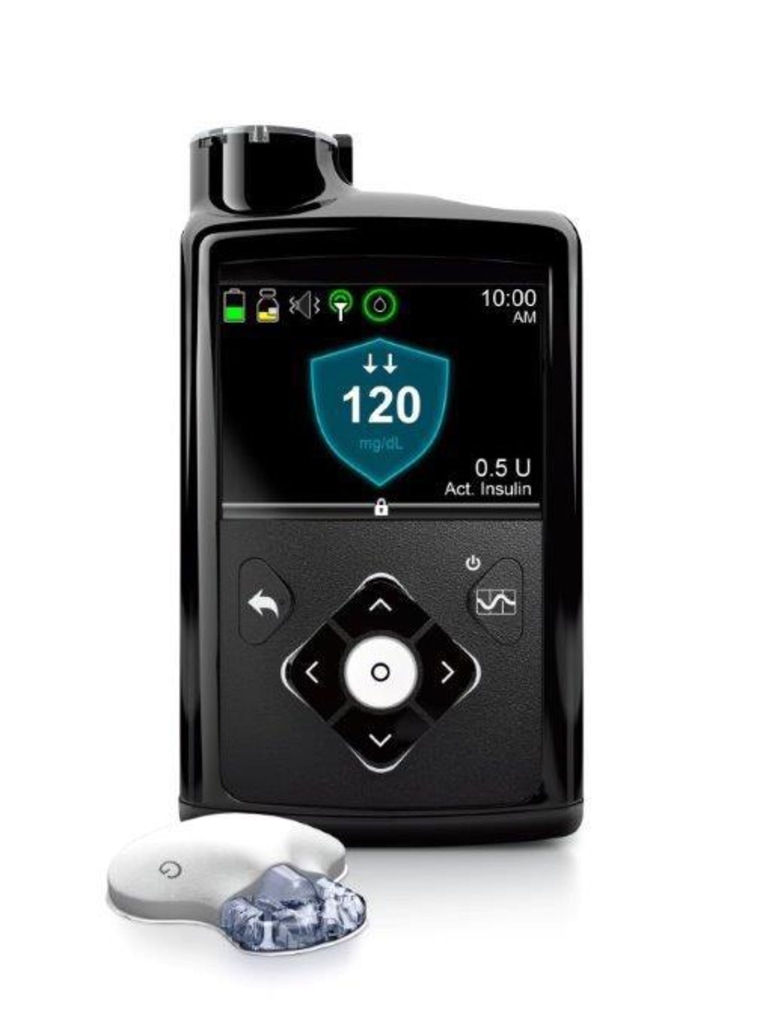
Medtronic first alerted patients about potential problems with the pumps in November, according to the FDA. In letters, Medtronic advised users to check the retainer rings on their pumps and said that if the reservoir compartment is not locking onto the pump or if the ring is loose, damaged or missing, they should stop using the pump immediately and contact their doctor to switch to another way to administer insulin.
If the reservoir is locking into place correctly, they can continue to use their pump. Checking whether the reservoir is locked in correctly needs to be done after every change, and especially if the reservoir is dropped. The pump should not be used if the reservoir or the ring are damaged.
If a patient has a pump with a faulty retainer ring, the letter advised contacting Medtronic customer service for a replacement pump through the Medtronic website or by calling the 24-hour Medtronic Technical Support line at 877-585-0166.
This is the second recall of Medtronic insulin pump devices in the last year. The company issued a recall in August because of cybersecurity risks linked to the remote controllers used to operate certain devices. The 500 series and Paradigm model remotes were discovered to be vulnerable to hacking in June.
CORRECTION (Feb. 13, 2020, 10:43 p.m. ET): An earlier version of this article inaccurately described insulin pumps as implanted devices. They are not implanted.
Follow NBC HEALTH on Twitter & Facebook.