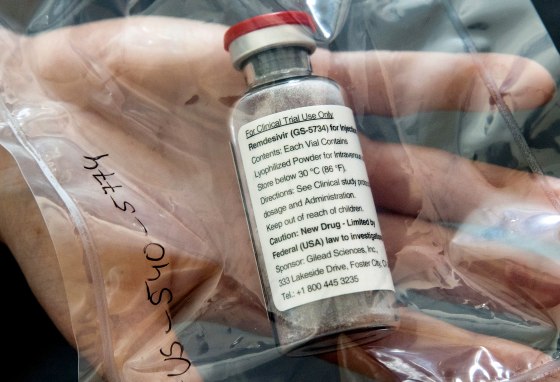
The only drug found so far to help treat COVID-19 may not work as well if it's paired with hydroxychloroquine, the Food and Drug Administration said Monday.
The FDA said it has become aware of a "potential drug interaction" between hydroxychloroquine and remdesivir, which "may result in reduced antiviral activity of remdesivir." The combination isn't recommended, according to the agency.
Full coverage of the coronavirus outbreak
Remdesivir isn't a cure for the coronavirus, but it has been shown in a clinical trial to help the sickest COVID-19 patients recover more quickly.
The FDA's announcement came the same day the agency rescinded its emergency use authorization for hydroxychloroquine to treat patients hospitalized with the coronavirus.

The latest warning, about the drug interactions, is based on a recent "non-clinical laboratory study," the FDA said, not on findings from a clinical trial of the drugs.
The FDA granted remdesivir an emergency use authorization in early May. It's authorized for use in hospitalized patients.
Download the NBC News app for full coverage of the coronavirus outbreak
The agency's statement also noted several potential side effects of remdesivir, including increased levels of liver enzymes, which may be a sign of inflammation or damage to cells in the liver. Allergic reactions are also possible.
"As we have done throughout the pandemic, the FDA continues to evaluate all of the emergency use authorizations issued and their related materials and will continue to make changes as appropriate based on emerging science and data," Dr. Patrizia Cavazzoni, acting director of the FDA's Center for Drug Evaluation and Research, said in a statement.