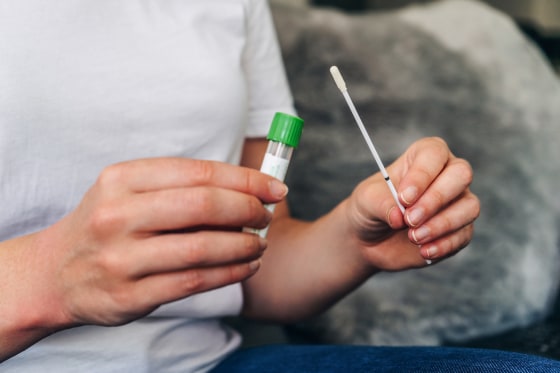
Federal guidelines are expanding the options for cervical cancer screenings beyond the often-disliked Pap smear.
According to new recommendations released Monday by the Health Resources and Services Administration (part of the Health and Human Services Department), women ages 30 to 65 with an average risk of cervical cancer can opt for a self-administered HPV test.
The test detects human papillomavirus, which causes cervical cancer — a disease that claims more than 4,000 lives each year in the U.S.
Per the new recommendations, women should get an HPV test every five years, either via self-swab or administered by a clinician. Doctors should still make Pap smears available, the guidelines say, though they’re no longer the preferred cervical cancer screening method for women ages 30 and up. HRSA still recommends Pap smears every three years for women ages 21 to 29.
“The guideline also requires most private insurance plans to cover screening and necessary follow-up testing without cost sharing, helping reduce out-of-pocket costs that can deter care,” HRSA told NBC News in a statement.
Insurance companies must adhere to the new screening guidelines by Jan. 1, 2027.
The new guidance reflects the latest research showing that HPV tests, compared to Pap smears, can increase the detection of abnormal cells in the cervix that may lead to cancer. Previous federal guidelines from 2016 recommended HPV tests and Pap smears every five years — or Pap smears every three years — for women ages 30 to 65.
Self-administered HPV tests involve inserting a plastic tube — similar to a tampon — into the vagina, then twisting the handle to swab for cells that can be examined in a lab. Pap smears, on the other hand, use a metal or plastic device called a speculum to widen the vaginal canal so a clinician can swab the cervix for cells.
Studies have found that self-swab HPV tests are similarly accurate to the ones administered by a clinician. Since 2024, the Food and Drug Administration has approved two self-swab tests that can be used in doctors’ offices, as well as an at-home test called the Teal Wand that can be ordered after a telehealth visit. The test is sold for nearly $250 out of pocket, though some insurance plans cover it.
In December, the American Cancer Society updated its guidance to endorse self-swab HPV tests every three years for women ages 25 to 65 — though it prefers a clinician-administered HPV test every five years.
“These recommendations are designed to maximize the benefits of early detection while minimizing potential harms from over-screening and unnecessary procedures,” said Dr. Robert Smith, senior vice president of early cancer detection science for the American Cancer Society.
While cervical cancer is highly preventable, rates are increasing year over year among women in their 30s and early 40s — perhaps as a result of delayed screenings or missing out on HPV vaccinations, which were approved in 2006. The vaccines can prevent more than 90% of cervical cancers, and routine screenings help detect HPV infections or precancerous cells.
In an editorial published Monday in the Journal of the American Medical Association, federal health officials said the new guidelines could help increase screening rates, particularly for women in rural areas or those who don’t have easy access to health clinics or doctor’s offices. Some women may also prefer the privacy of self-swab tests or find them more comfortable than other forms of screening, they said.
A quarter of women ages 21 to 65 in the U.S. are not up to date on cervical cancer screening, and screenings among that group declined after the pandemic — from 47% in 2019 to 41% in 2023.
“By reducing testing barriers, expanding choice, empowering women, and eliminating patient cost sharing … [the] guidelines for cervical cancer screening are a powerful step forward for women’s health across the US,” the health officials wrote.